UNIT 10: CHEMICAL BONDING(8 DAYS)
Believe it or not, the chemical properties of almost any substance or material in the world depend upon the chemical bonds that make it up. If it weren't for chemical bonds, every material in the world would have to be one of the 118 elements in the Periodic Table. Instead, atoms from these elements bond together to create chemical compounds. |
NC STANDARDS
PSc.2.2.2 Infer the type of chemical bond that occurs, whether covalent, ionic, or metallic, in a given substance.
PSc.2.2.3 Predict chemical formulas and names for simple compounds based on knowledge of bond formation and naming conventions.
PSc.2.2.3 Predict chemical formulas and names for simple compounds based on knowledge of bond formation and naming conventions.
|
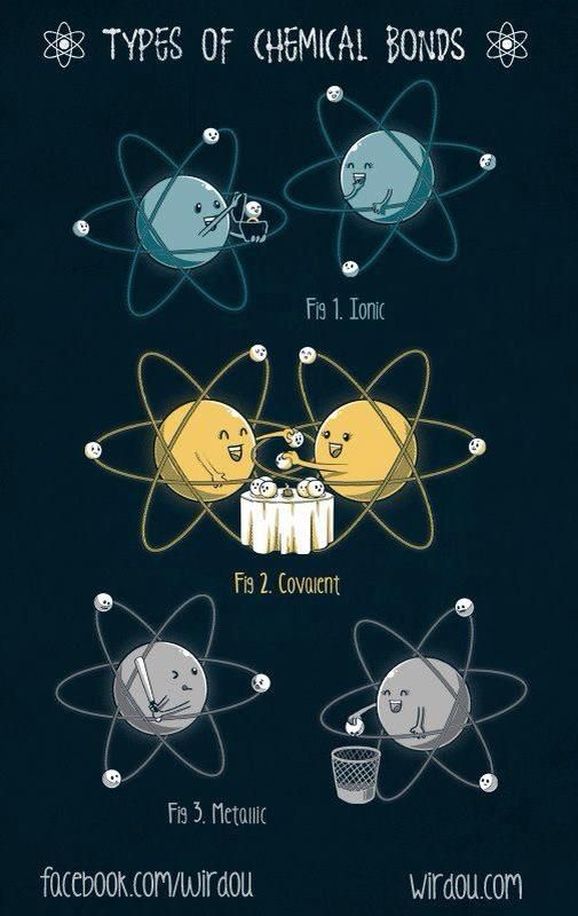
DAY 1: INTRODUCTION TO CHEMICAL BONDING
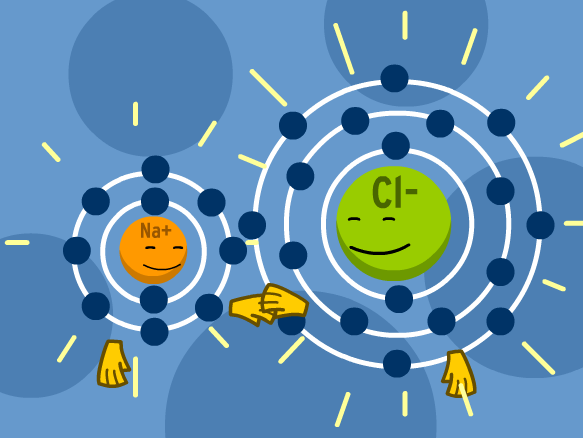
DAY 2: IONIC BONDING
The packet below covers Ionic & Covalent Bonds. We will work through this packet together in class.
|
| ||||||||||||||||||||||||
|
DAY 3: COVALENT BONDING
Work on PACKET
DAY 4: IONIC & COVALENT PRACTICE
|
| ||||||
DAY 5: WRITING & NAMING COMPOUNDS
Work on PACKET
DAY 6: METALS
DAY 7: REVIEW
|
Chemical Bonding Review Study Guide on QUIA
(Same as the pdf to the left) | ||||||
DAY 8: UNIT TEST
LABS